11. Medication Codes
Medication Codes
ND320 AIHCND C01 L02 A09 Medication Codes V2
Medication Code Key Points
NDC: National Drug Code
In this section, we discussed the NDC Codes. These codes have been in place since 1972, and are maintained by the FDA.
NDC Code Structure
- 10- to 11-digit code
- multiple configurations
- 3 Parts
- Labeler: Drug manufacturer
- Product code: the actual drug details
- Package code: form and size of medication
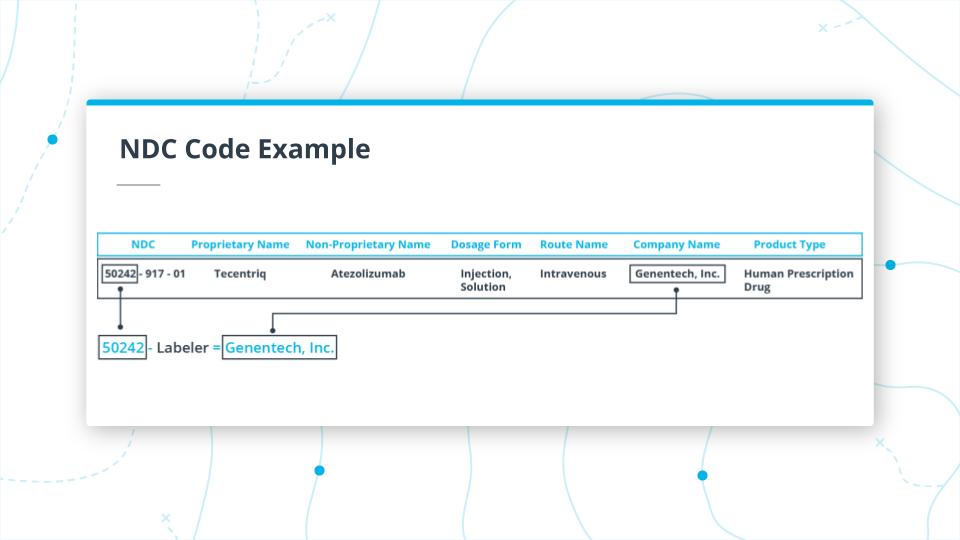
In the image above you, can see the first part of the NDC code for Tecentriq.
The first part of the code 50242 is the Labeler, which maps to the manufacturer of the drug (which in this case is Genentech, Inc).
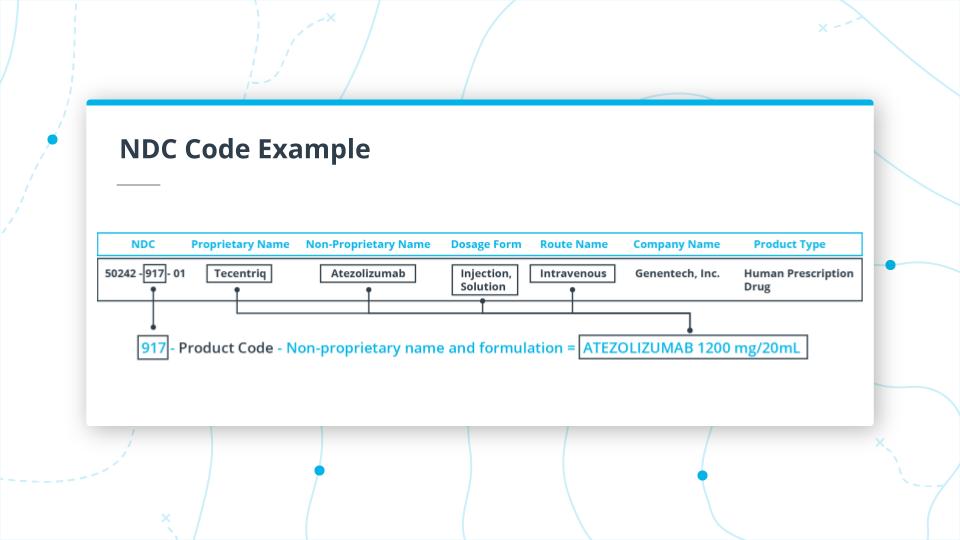
NDC Product Code
In the example above, we are looking at the middle section of the code 917.
917 is the Product Code. In this case, it takes the unpronounceable, Non-Proprietary Name, ATEZOLIZUMAB 1200mg/20ml, and maps it to the Proprietary Name, Tecentriq.
It also indicates that the drug dosage form is Injection, Solution and the route of administration is Intravenous.
It is important to note that, you'd have to use the Non-Proprietary Name for generic-wise grouping of all drugs.
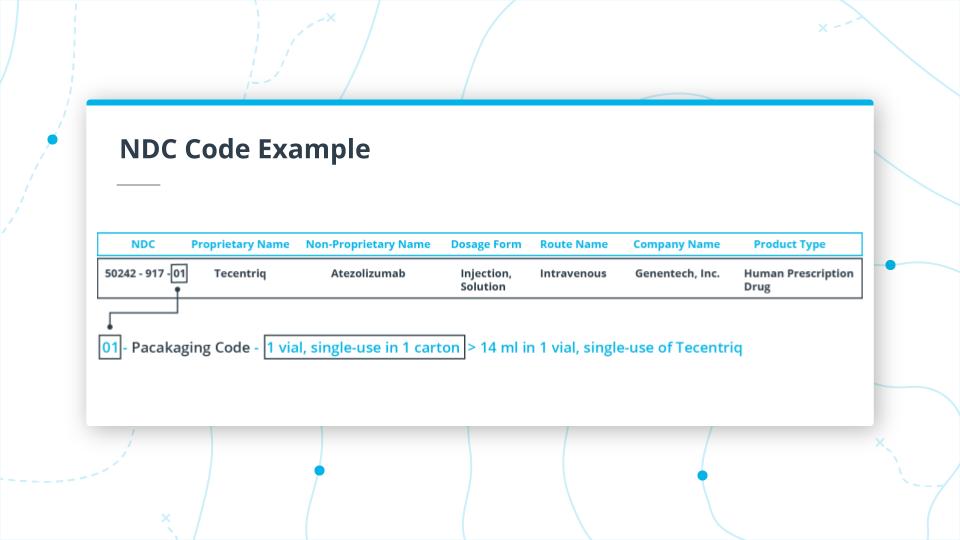
NDC Packaging Code
Finally, the last two digits 01.
This is the packaging code, and in this instance indicates that the package is a 14ml single vial in 1 carton for single use.
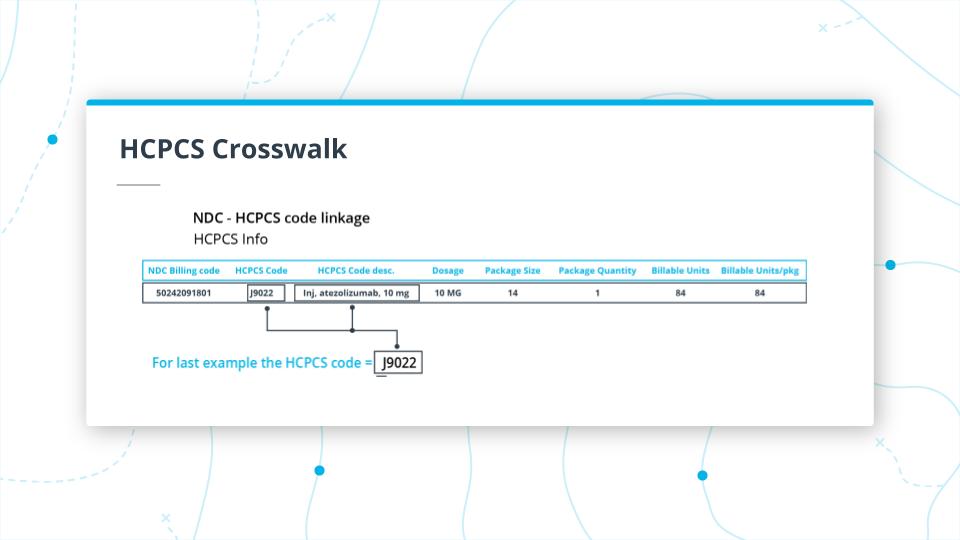
HCPCS Crosswalk
Crosswalk
Crosswalk: A connection between two different code sets or versions of drugs in the same code set.
A common term you will hear for medical code sets is a crosswalk.
For NDC codes we can connect them to HCPCS codes and depending on the data source you are looking at there may be a mapping from these codes.
For the previous example, there is a crosswalk between the Tecentriq NDC code and the HCPCS code J9022. Something to note is that the HCPCS code starts with a J and the “J” codes tend to be drugs that are injected but by definition, they are drugs that are not taken orally. You can see that the HCPCS code maps some info from the NDC code.
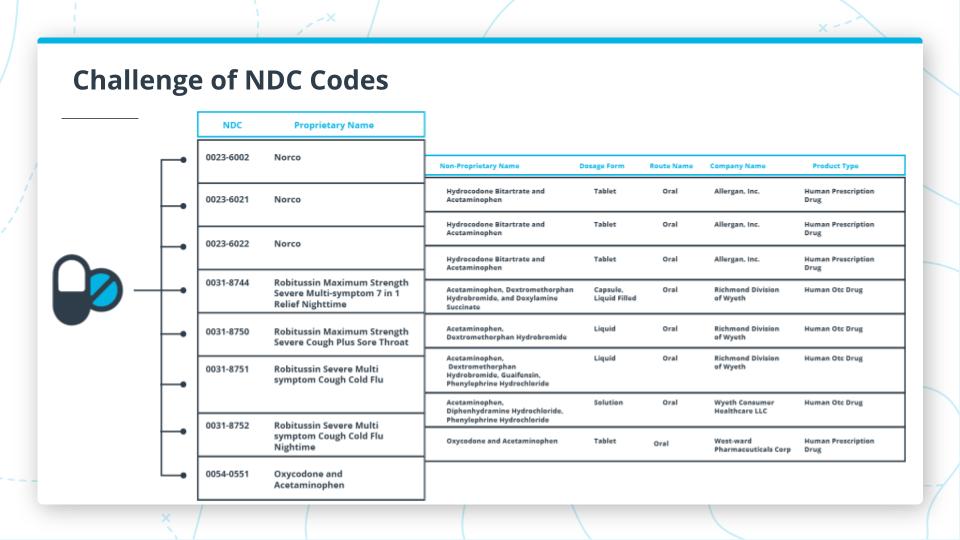
NDC Code Challenges
Challenge with NDC Codes
One of the major challenges with NDC codes is to normalize or group codes by common types of drugs. For example, let’s take acetaminophen. You would think this would be relatively straightforward to group whenever someone has the NDC code for this drug. However, as you can see from this table of only a sample of the results from NDC List that there many drug codes that could contain acetaminophen with other drugs too. This can make drugs very difficult to deal with.
Additional Resources:
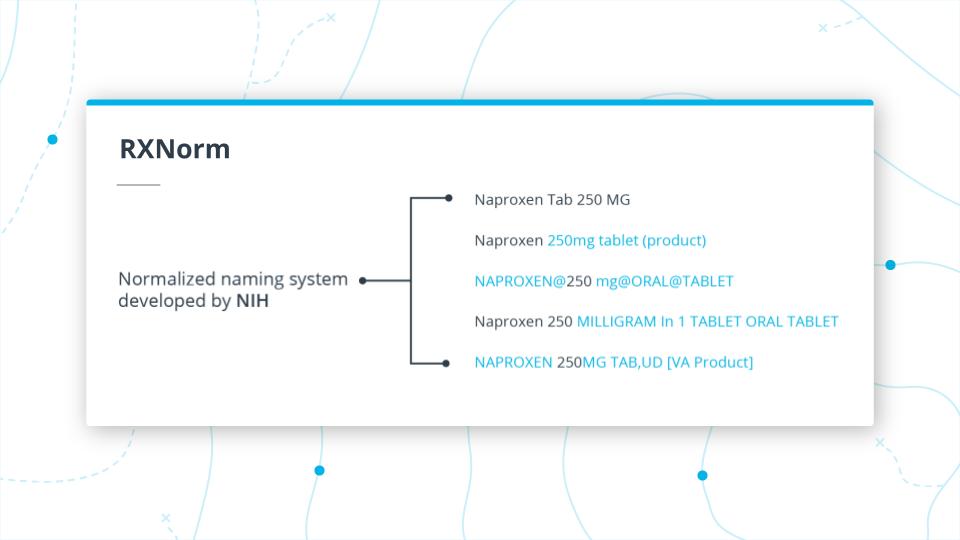
RXNorm Example
RXNorm
To address the problem just mentioned, the NIH developed a normalized naming system called RXNorm, which does what its name implies and groups medication together. This is important because providers, pharmacies, payers all send EHR records with this data but might use different names and it becomes difficult to communicate between different systems.
To illustrate this issue, take a look at a drug Naproxen and the just a few examples of different names of naproxen that is the same thing,
While there is a crosswalk between NDC codes and RXNorm, there are still some issues. Depending on the system you are dealing with, it could use one or the other code set.
Additional Resources
NDC Codes Quiz
SOLUTION:
- The NDC code 0573-0154 is for Advil.
- Advil is also known as ibuprofen and is taken orally.
- Capsaicin has an NDC code of 64058-113
- A crosswalk connects two different versions of a drug.
- The last two numbers indicate the packaging for a drug.
NDC Codes
QUESTION:
Use ndclist.com to help you answer this question.
Who makes the drugs that start with 0378?
What drug do they make with the code 4154?
What is this product's dosage form?
ANSWER:
Hopefully, you figured out that the drug manufacturer is Mylan Pharmaceuticals Inc. and they make the drug Tramadol Hydrochloride and the dosage form is a tablet, extended-release. Great job if you figured that out!
NDC Codes Continued
SOLUTION:
- The drug's main active ingredient is ibuprofen.
- Its name is Member's Mark Ibuprofen.
- It is made by Sam's West Inc which is the owner of Sam's Club.